In the field of drug discovery, Signal Transducer and Activator of Transcription 6 (STAT6) has long been a challenging "undruggable" target for countless scientists. As the core downstream hub of the IL-4/IL-13 signaling pathway, it plays a pivotal role in Th2-type inflammatory diseases such as asthma and atopic dermatitis. Among various intervention strategies, only promoting the degradation of STAT6 holds the potential to completely block the IL-4/IL-13 signaling pathway.
Most members of the STAT family are considered "difficult-to-drug" targets due to a lack of binding pockets and the redundant functions among STAT proteins, where blocking their activity may lead to unexpected toxic side effects. It wasn't until the rise of Targeted Protein Degradation (TPD) technology, particularly PROTAC technology, that a dawn broke through this darkness. Kymera Therapeutics' pioneering STAT6 degrader, KT-621, is the first STAT6-targeted degrader to advance into Phase 2 clinical trials. On December 8, 2025, BroADen Phase 1b data was announced, showing that the oral PROTAC degraded STAT6 by 98% (blood) and 94% (skin), with a reduction in Thymus-and Activation-Regulated Chemokine (TARC) levels matching that of Dupixent. Significant improvements in pruritus and skin lesions were observed, with zero serious adverse events. Kymera has now initiated the BROADEN2 Phase IIb clinical trial, which aims to evaluate the efficacy of KT-621 (an oral, highly selective, potent STAT6 degrader) in treating patients with moderate-to-severe Atopic Dermatitis (AD).
The breakthrough of KT-621 stems from precise target selection and an innovative mechanism of action. STAT6 is a key transcription factor in the IL-4/IL-13 pathway, a pathway whose importance in type 2 inflammatory diseases (including AD, asthma, chronic spontaneous urticaria, etc.) has been validated by the clinical success of Dupixent. Unlike Dupixent, which targets upstream receptors in the pathway, KT-621 directly acts on intracellular STAT6, utilizing PROTAC technology to achieve complete degradation of the target rather than the inhibitory effect of traditional small molecules. With its triple advantages of being oral, delivering biologic-level efficacy, and demonstrating a potentially superior safety profile, KT-621 has the potential to disrupt the current biologics-dominated market landscape, offering a new treatment paradigm for over 130 million potential patients with type 2 inflammatory diseases worldwide.
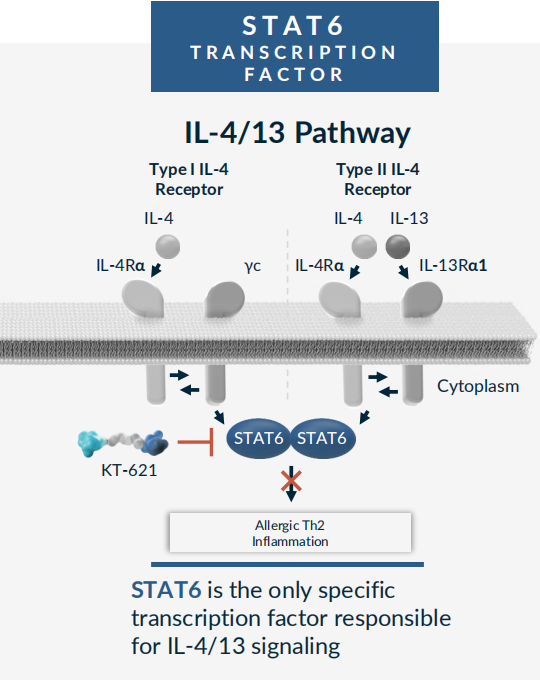
Figure 1. Schematic diagram of the STAT6 and IL-4/13 signaling pathway
How to accurately and efficiently identify PROTAC molecules from a vast sea of compounds is one of the critical steps towards success. The core challenge lies in rapidly, accurately, and reliably assessing whether a PROTAC molecule successfully "brings together" the target protein (e.g., STAT6) and an E3 ubiquitin ligase to form a stable "ternary complex." This is the decisive step for protein degraders to exert their effect.
To address this core challenge, we proudly introduce the STAT6/CRBN PROTAC Binding Assay Kit specifically designed for STAT6 degrader development. It is not just a kit; it is your precise navigation system for conquering the STAT6 research fortress.
Among various biophysical detection technologies, Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) has become the gold standard for PROTAC screening and mechanism studies due to its unique advantages. Compared to traditional methods that are time-consuming and require specialized instrumentation, TR-FRET offers a homogeneous (no-wash), rapid, and high-throughput assay solution.
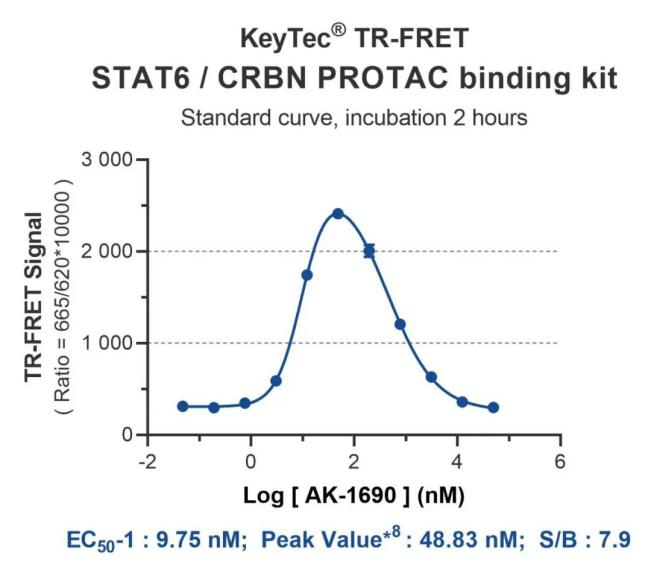
The detection principle is illustrated in the figure below. The KeyTec® TR-FRET Solar Eu-conjugated anti-Tag1 antibody specifically recognizes the Tag1-STAT6 protein, while the KeyTec® TR-FRET LA-conjugated anti-Tag2 antibody specifically recognizes the Tag2-CRBN/DDB1 protein complex. The Tag1-STAT6 protein and the Tag2-CRBN/DDB1 protein complex can form a ternary complex mediated by the positive control PROTAC molecule, AK-1690. This brings the Solar Eu and LA into close proximity, enabling Fluorescence Resonance Energy Transfer between the donor and acceptor upon excitation by an external light source. Measuring the signal intensity at a specific wavelength (665nm) allows for the determination of the binding between STAT6 and CRBN/DDB1 mediated by the PROTAC molecule.
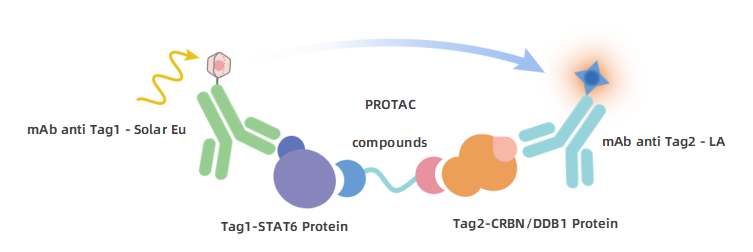
Figure 2. STAT6/CRBN PROTAC binding detection principle
AK-1690 is a potent and highly selective STAT6 degrader. In MV411 human leukemia cells, Peripheral Blood Mononuclear Cells (PBMCs), and L1236 Hodgkin's lymphoma cells, AK-1690 significantly reduced STAT6 protein levels without markedly affecting other STAT members. In the MV411 cell line, AK-1690 demonstrated a DC50 of 1 nM and a Dmax exceeding 95%; in PBMCs, it showed a DC50 of 80 nM and a Dmax over 90%. In the L1236 cell line, AK-1690 effectively induced depletion of both total STAT6 and phosphorylated STAT6, while having no impact on other STAT proteins.