In our previous issue, we introduced VKEY-BIO's new TR-FRET Effector Function Analysis Kits (CD16a/CD64) to aid in the assessment of antibody ADCC/ADCP functions. This issue focuses on another core pharmacokinetic property of antibody drugs——their in vivo circulation levels and half-life. The key molecule governing this critical property is the Neonatal Fc Receptor (FcRn).
The neonatal Fc receptor (FcRn) is a specific receptor for immunoglobulin G (IgG), composed of a heavy chain and a light chain non-covalently bound. FcRn has an intracellular domain, a transmembrane domain, and three extracellular functional domains: α1, α2, and α3. These three extracellular domains, together with the light chain (β2-microglobulin), form the interaction site for FcRn and IgG (Figure 1).
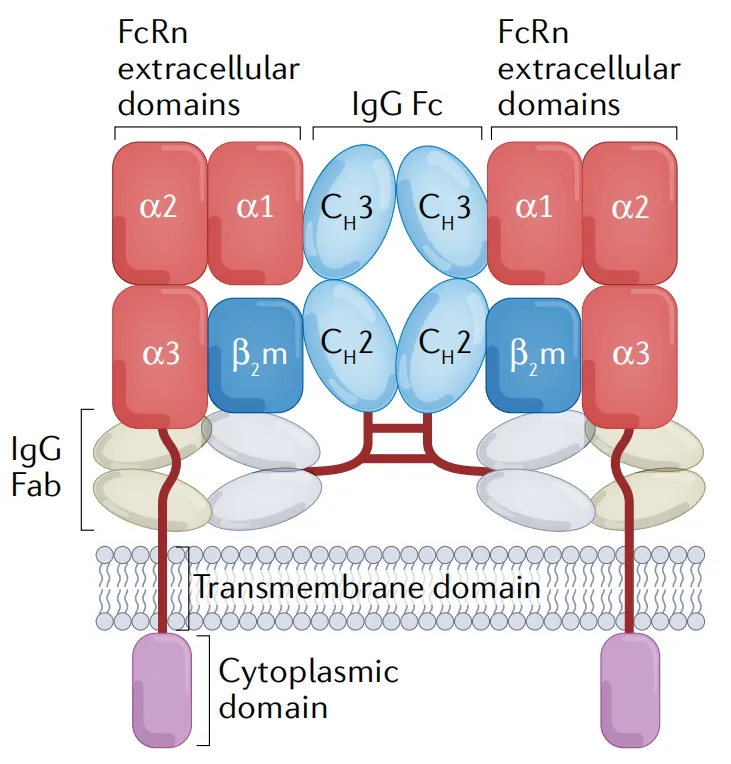
Figure 1. Structure of FcRn and its ligands
(doi: 10.1038/s41577-022-00821-1)
The binding between FcRn and IgG is pH-dependent, forming the basis for FcRn's role in maintaining homeostasis of plasma IgG levels (Figure 2) and the transcytosis of IgG (Figure 3). Immunoglobulin IgG is an essential soluble component in the adaptive immune response, protecting the body from infection. Compared to other immunoglobulins, IgG is notable for its high circulating levels, long half-life, and ability to be transferred from mother to offspring.
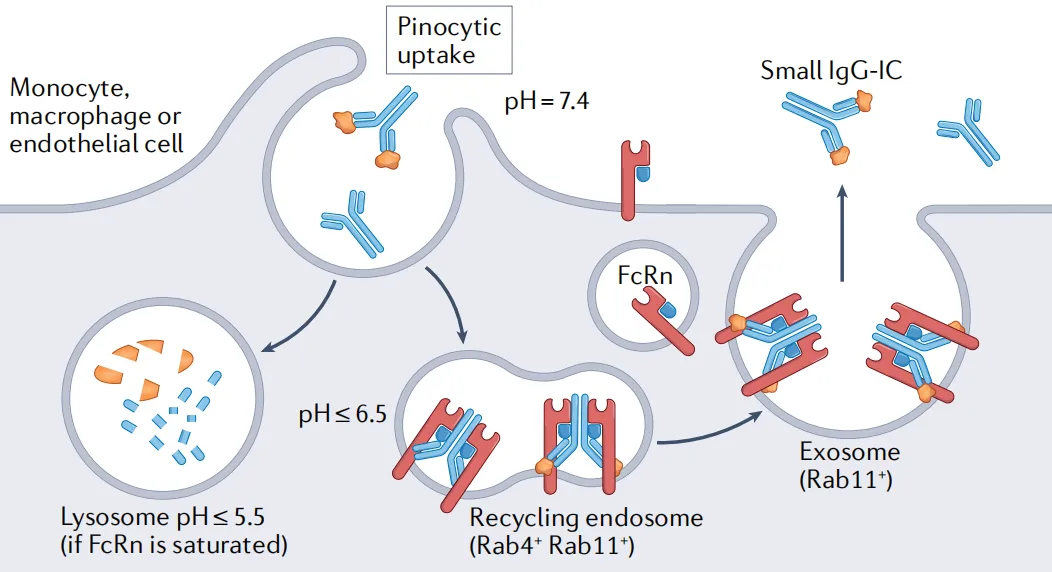
Figure 2. FcRn-mediated recycling of IgG or IgG immune complexes
(doi: 10.1038/s41577-022-00821-1)
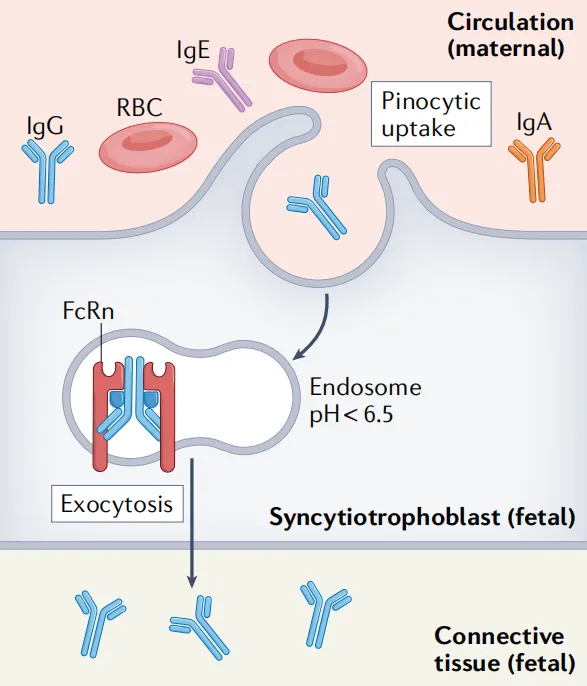
Figure 3. FcRn-mediated transfer of maternal IgG to offspring
(doi: 10.1038/s41577-022-00821-1)
(1) In autoimmune diseases, anti-FcRn antibodies can promote the degradation of pathogenic IgG by competitively binding to FcRn;
(2) In infectious diseases, increasing the affinity of therapeutic antibodies for FcRn can extend drug half-life, while fusing viral antigens to the Fc fragment of IgG can elicit local mucosal immune responses for disease prevention and treatment;
(3) In cancer, the Fc region (the FcRn binding domain) can serve as a carrier for anticancer drugs to enable efficient delivery, and FcRn itself can serve as a prognostic predictor for cancer patients.
Leveraging its self-developed TR-FRET technology platform, VKEY-BIO has developed the KeyTec® TR-FRET Human FcRn Binding Assay Kit. This tr fret assay kit is specifically designed for the efficient screening of IgGs binding to Human FcRn and is an ideal tool for assessing the in vivo half-life potential of antibody drug candidates and for the high-throughput screening of FcRn inhibitors.
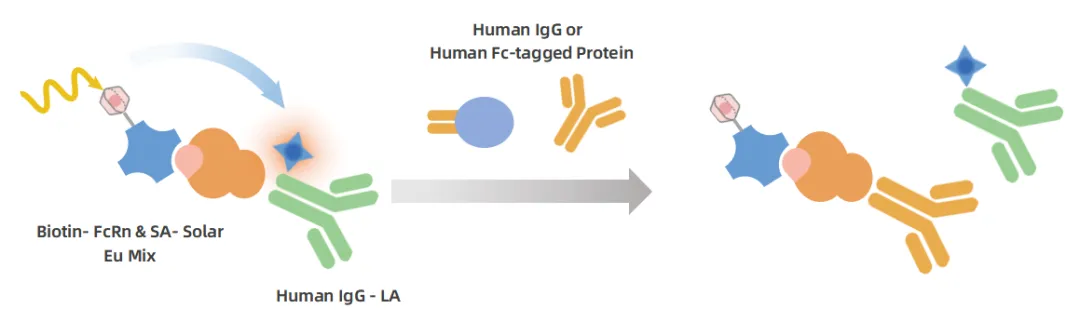
Figure 4. Schematic diagram of the KeyTec^®^ TR-FRET FcRn Binding Assay principle
Ultra-Simple Operation: Homogeneous reaction system, completed in three steps: "Add-Incubate-Read", no washing required. The dilution buffer offers excellent compatibility.
Excellent Performance: Low background, high signal window (S/B > 10), ensuring accurate and reliable results.
Flexible Throughput: Supports direct miniaturization from 96/384-well plates to high-throughput screening modes, such as 384-well plates and 1536-well plates.
Time-Efficient & Highly Effective: Stable signals allow for flexible readout times or screening scales (1h to overnight incubation).
Kit Performance:
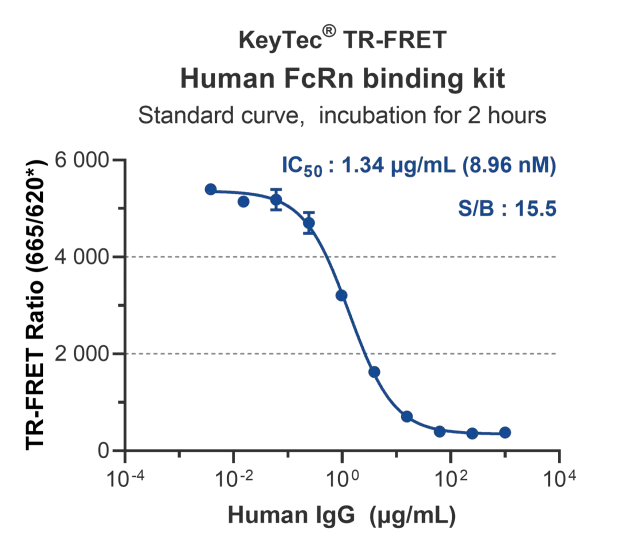
Standard curve serial dilution shows: The kit has low background signal (200~300) and a high detection window (>10).
Accurately Distinguishes Affinity of Different IgG Subtypes:
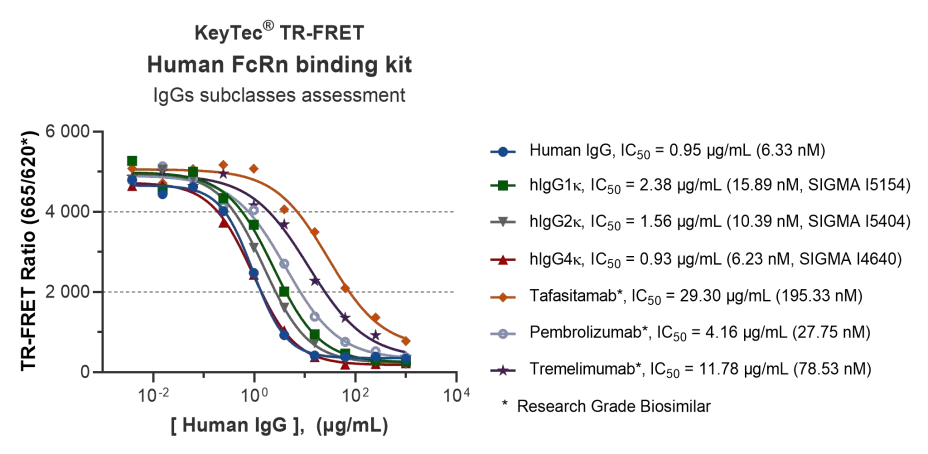
The kit can clearly distinguish the differences in binding affinity to FcRn between different human IgG subtypes (hIgG1κ, hIgG2κ, hIgG4κ) (Affinity order: hIgG4κ > hIgG2κ > hIgG1κ).
Incubation Time:
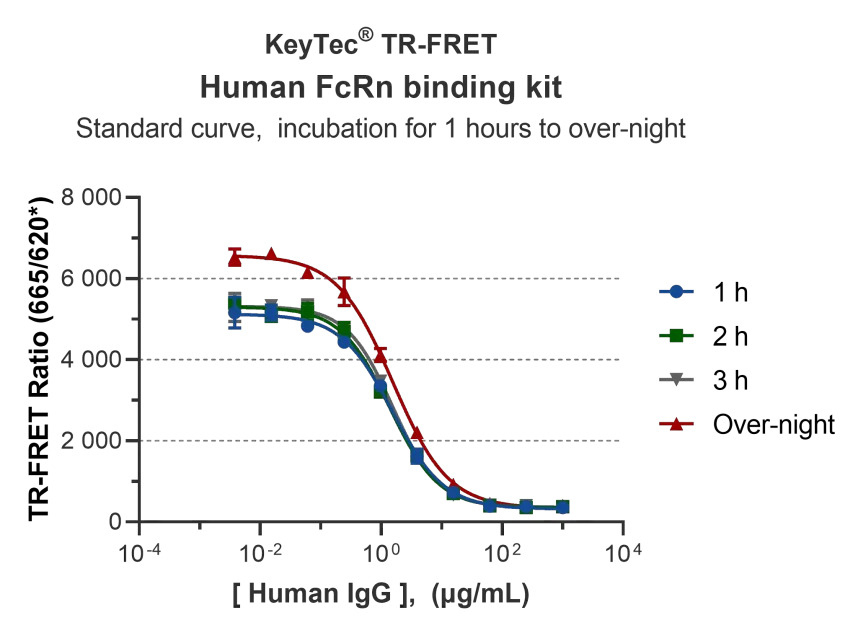
Detection signal window under different incubation times: A significant detection window as high as 15-fold can be achieved after just 1 hour of incubation, meeting the needs of rapid screening in drug discovery reagents applications.
⭐Note:
Sample matrix (e.g., serum concentration) can interfere with the assay system. It is recommended to dilute samples at least 4-fold using the Assay Diluent Buffer provided in the kit before detection. When preparing the standard curve, please use the same matrix solution as used for the samples.
