In the field of autoimmune disease treatment, TL1A (TNF-like ligand 1A) and its receptors DR3 (Death Receptor 3) and decoy receptor DcR3 (Decoy Receptor 3) have emerged as promising new targets. As global pharmaceutical companies increasingly focus on drug development targeting the TL1A/DR3 pathway, the efficient and accurate screening of inhibitors that block the binding of TL1A to DR3 or DcR3 has become a critical step in the process.To address this need, VKEY-BIO proudly introduces two innovative products—the KeyTec® TR-FRET Human TL1A/DR3 Binding Kit and the KeyTec® TR-FRET Human TL1A/DcR3 Binding Kit—leveraging advanced TR-FRET technology to provide highly efficient and reliable bioassay kits for drug development and precise evaluation of autoimmune therapeutics.
TL1A, a member of the tumor necrosis factor (TNF) superfamily, activates downstream signaling pathways by binding to DR3, regulating immune cell proliferation, apoptosis, and inflammatory cytokine release. It plays a pivotal role in diseases such as ulcerative colitis, Crohn’s disease, and rheumatoid arthritis. Meanwhile, DcR3 acts as a decoy receptor, competitively binding to TL1A and blocking its interaction with DR3, thereby suppressing excessive immune responses.
Currently, several TL1A-targeted drugs have entered clinical trials worldwide, including Roche’s Afimkibart and MSD’s Tulisokibart, both in Phase III, while China’s Sansheng Guojian has advanced its TL1A monoclonal antibody, SSGJ-627, into clinical applications. However, existing tools for screening TL1A/DR3 or TL1A/DcR3 binding inhibitors often suffer from low sensitivity and cumbersome procedures. VKEY-BIO’s new assay kits fill this technological gap.
Human TL1A/DR3 Binding Kit
Principle: Utilizing competitive TR-FRET technology, Solar Eu-labeled streptavidin forms a donor by pre-binding with biotinylated TL1A, while LA-labeled DR3 serves as the acceptor. In the presence of an inhibitor, DR3 and TL1A cannot bind, leading to a reduction in TR-FRET signal intensity, which inversely correlates with inhibitor concentration.
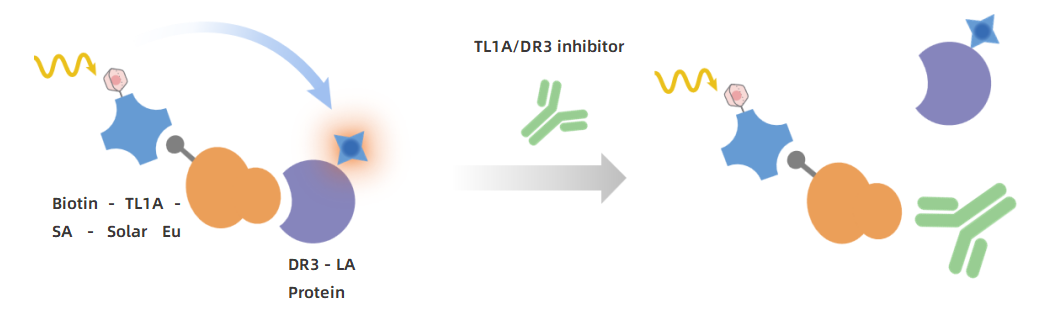
KeyTec® TR-FRET Human TL1A/DR3 Binding Kit Principle
Advantages:
High Sensitivity: Capable of detecting inhibitor activity at nanomolar (nM) levels, with a signal-to-background (S/B) ratio of up to 4-fold.
Excellent Reproducibility: High consistency across repeated experiments (CV% < 10%).
Simple Operation: A 30-minute pre-incubation significantly enhances the detection window, supports room temperature incubation, and eliminates the need for complex washing steps.
Human TL1A/DcR3 Binding Kit
Principle: Solar Eu-labeled anti-Tag1 antibody recognizes Tag1-TL1A, forming energy transfer with LA-labeled DcR3. Inhibitors prevent DcR3 and TL1A binding, reducing TR-FRET signal.
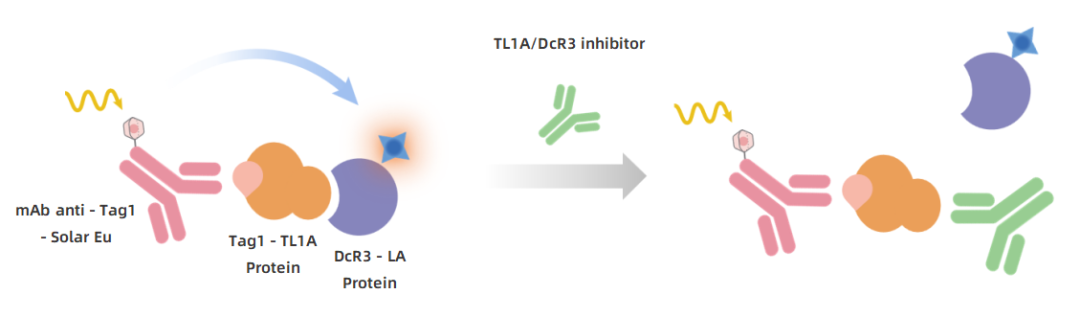
KeyTec® TR-FRET Human TL1A/DcR3 Binding Kit Principle
Advantages:
Ultra-High S/B Ratio: S/B ratio reaches 10-fold, outperforming competitors.
Flexible Operation: Supports pre-mixed Donor/Acceptor addition to minimize operational errors; pre-incubation does not affect results, making it ideal for high-throughput screening.
Broad Applicability: Compatible with various inhibitors, such as Tulisokibart.
Human TL1A/DR3 Binding Kit
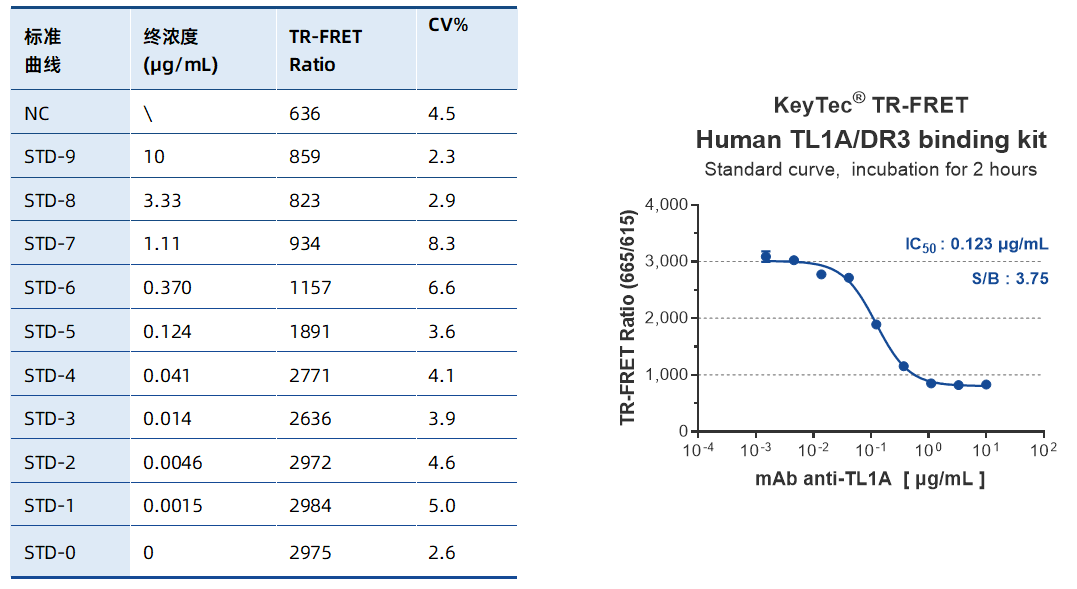
Sample Test Data
High Sensitivity: IC50 of 0.123 μg/mL, demonstrating precise detection of low-concentration inhibitors.
Outstanding Stability: CV% < 10%, ensuring high reproducibility for long-term drug screening needs.
Wide Dynamic Range: Covers concentrations from 10 μg/mL to 0.0015 μg/mL, suitable for inhibitors with varying affinities.
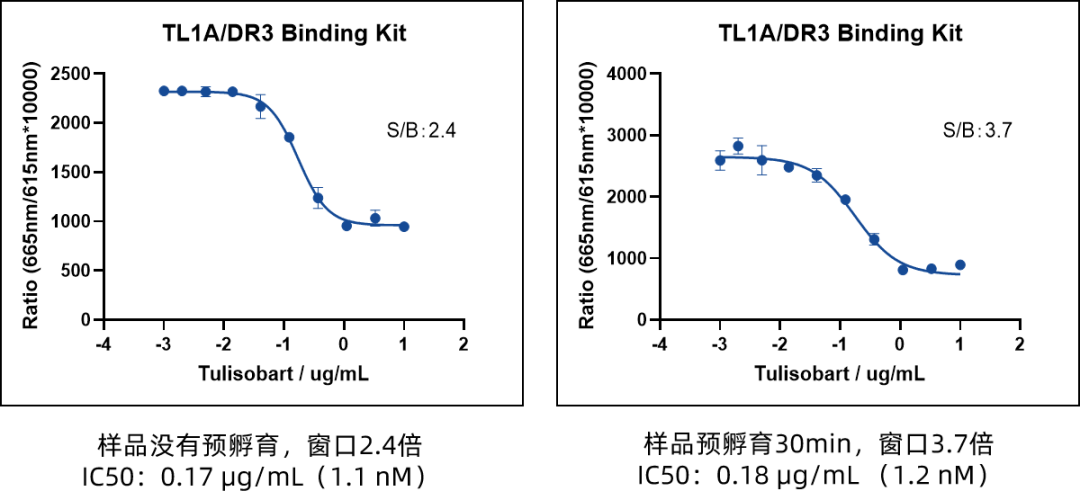
Impact of Pre-Incubation with TL1A on Detection Window
- Pre-incubation significantly improves the detection window (S/B increases from 2.4 to 3.7); a 30-minute pre-incubation is recommended for enhanced data quality.
- Stable IC50 (0.17 μg/mL vs. 0.18 μg/mL) confirms that pre-incubation does not alter inhibitor binding properties but optimizes signal intensity.
Human TL1A/DcR3 Binding Kit
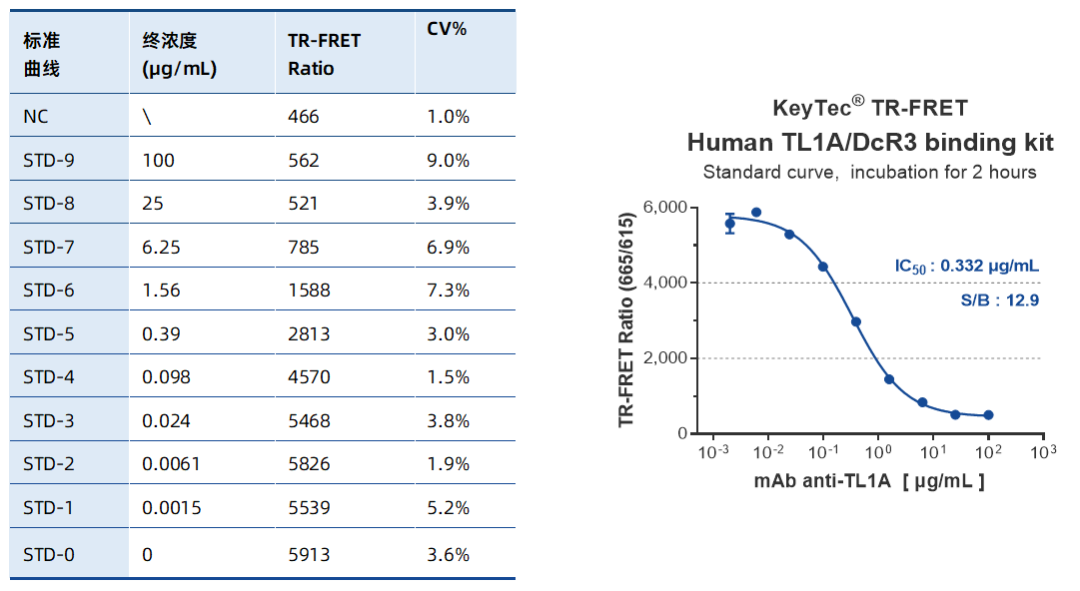
Sample Test Data
Ultra-High S/B Ratio: Broad dynamic signal range (466–5913), ideal for low-abundance samples.
High Precision: CV% < 10%, ensuring excellent data stability.
IC50 Validation: Tulisokibart IC50 of 0.332 μg/mL confirms the assay’s reliability and accuracy.
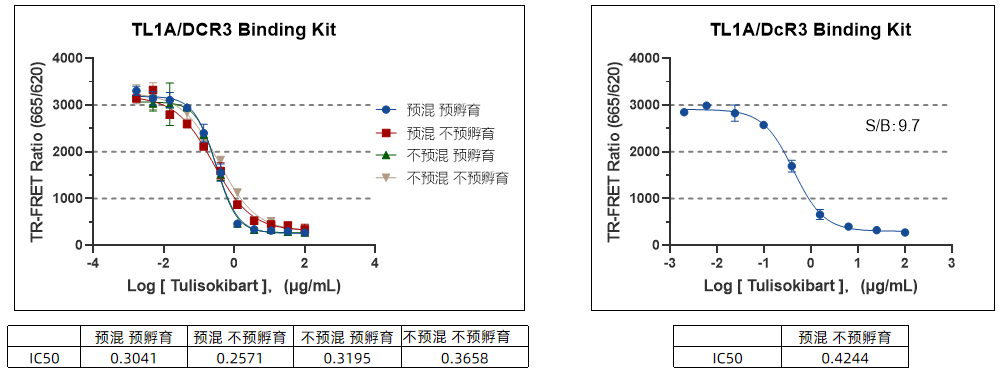
Impact of Pre-Incubation with TL1A on Detection Window
High Operational Flexibility: Pre-mixed or sequential addition does not affect results, accommodating different experimental preferences.
Reduced Human Error: Pre-mixed mode is recommended to streamline the process and minimize operational variability.
Drug Screening: Rapid evaluation of TL1A/DR3 or TL1A/DcR3 inhibitor binding activity.
Mechanistic Studies: Elucidating the molecular mechanisms of TL1A-receptor interactions.
Preclinical Evaluation: Providing reliable potency assessment tools for antibody drugs like Tulisokibart.